MONTREAL, June 18, 2021 / Spinologics Inc. / Spino Modulation Inc., a subsidiary of
Spinologics Inc., announced today that the U.S. Food and Drug Administration (FDA) has
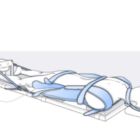
granted the company a Breakthrough Device Designation for its MIScoli™ system, an
innovative vertebral body tethering (VBT) device to treat scoliosis in young adolescents.